|
||||||
|
UniGene Name: sp_v3.0_unigene135975
Length: 166 nt
This UniGene was originaly assembled in antisense
ACE File: antisense
Fasta: sense
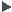 UniGene Fasta (sense) UniGene Fasta (sense)
|
|---|
| >sp_v3.0_unigene135975
T |
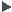 Ace file of the UniGene sp_v3.0_unigene135975 (antisense) Ace file of the UniGene sp_v3.0_unigene135975 (antisense)
|
|---|
If you push the Download ace button, you will download an ace file of this UniGene.
To watch this ACE file you will need an ACE viewer program as Tablet:
Go to the Tablet ACE viewer Web
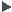 Annotations Annotations |
|---|
| Source | Descriptions | Term | Type | e value | Identity |
|---|---|---|---|---|---|
| AutoFact | Putative sinapyl alcohol dehydrogenase (Fragment) n=1 Tax=Ginkgo biloba RepID=E5F5P7_GINBI | - | - | 5.0e-13 | 74% |
| FL-Next | tr=Putative uncharacterized protein; Picea sitchensis (Sitka spruce) (Pinus sitchensis). | - | - | 0.0 | 90% |
| Sma3 | Cinnamyl alcohol dehydrogenase | - | - | 6.24e-38 | - |
| Source | ECs | Term | Type | e value | Identity |
|---|---|---|---|---|---|
| Sma3 | Cinnamyl-alcohol dehydrogenase. | EC:1.1.1.195 | - | 1.934e-14 | - |
| Source | KEGGs | Term | Type | e value | Identity |
|---|---|---|---|---|---|
| Sma3 | Phenylpropanoid biosynthesis | 00940 | 1.934e-14 | % | |
| Sma3 | Biosynthesis of phenylpropanoids | 01061 | 1.934e-14 | % | |
| Sma3 | Metabolic pathways | 01100 | 1.934e-14 | % | |
| Sma3 | Biosynthesis of secondary metabolites | 01110 | 1.934e-14 | % | |
| Sma3 | Mannitol dehydrogenase. | EC:1.1.1.255 | - | 0.0 | - |
| Source | Gene names |
|---|---|
| Sma3 | AT2G21730; AT2G21890; AT4G37980; AT4G39330; At1g72680; At2g21730; At2g21890; At4g37980; At4g37990; At4g39330; AtCAD1; CAD; CAD1; CAD2; CAD3; CAD7; CAD8; CADL1; CADL10; CADL11; CADL2; CADL3; CADL4; CADL5; CADL6; CADL7; CADL8; CADL9; CADb; CADb-1; CADb-2; C |
| Source | GOs | Term | Type | e value | Identity |
|---|---|---|---|---|---|
| Sma3 | cytoplasm | GO:0005737 | Cellular Component | 0.0 | - |
| Sma3 | apoplast | GO:0048046 | Cellular Component | 0.0 | - |
| Sma3 | protein binding | GO:0005515 | Molecular Function | 0.0 | - |
| Sma3 | zinc ion binding | GO:0008270 | Molecular Function | 0.0 | - |
| Sma3 | oxidoreductase activity | GO:0016491 | Molecular Function | 0.0 | - |
| Sma3 | oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | GO:0016616 | Molecular Function | 0.0 | - |
| Sma3 | cinnamyl-alcohol dehydrogenase activity | GO:0045551 | Molecular Function | 0.0 | - |
| Sma3 | mannitol dehydrogenase activity | GO:0046029 | Molecular Function | 0.0 | - |
| Sma3 | cofactor binding | GO:0048037 | Molecular Function | 0.0 | - |
| Sma3 | response to bacterium | GO:0009617 | Biological Process | 0.0 | - |
| Sma3 | oxidation-reduction process | GO:0055114 | Biological Process | 0.0 | - |
| Source | InterPros | Term | Type | e value | Identity |
|---|---|---|---|---|---|
| Sma3 | Alcohol dehydrogenase superfamily, zinc-type | IPR002085 | - | 0.0 | - |
| Sma3 | Alcohol dehydrogenase, zinc-type, conserved site | IPR002328 | - | 0.0 | - |
| Sma3 | Sugar transporter, conserved site | IPR005829 | - | 0.0 | - |
| Sma3 | D-isomer specific 2-hydroxyacid dehydrogenase, NAD-binding | IPR006140 | - | 0.0 | - |
| Sma3 | TonB box, conserved site | IPR010916 | - | 0.0 | - |
| Sma3 | Alcohol dehydrogenase, C-terminal | IPR013149 | - | 0.0 | - |
| Sma3 | Alcohol dehydrogenase GroES-like | IPR013154 | - | 0.0 | - |
| Sma3 | NAD(P)-binding domain | IPR016040 | - | 0.0 | - |
| Sma3 | 4Fe-4S ferredoxin, iron-sulphur binding, conserved site | IPR017900 | - | 0.0 | - |
| Source | Species | ID | Description | e value | Identity |
|---|---|---|---|---|---|
| ATG | Arabidoptis thaliana | AT1G72680.1 | ATCAD1, CAD1 cinnamyl-alcohol dehydrogenase chr1:27359346-27360876 REVERSE LENGTH=355 | 3.0e-11 | 71% |
| RefSeq | Arabidopsis thaliana | NP_177412.1 | putative cinnamyl alcohol dehydrogenase 1 [Arabidopsis thaliana] | 4.0e-11 | 71% |
| RefSeq | Populus trichocarpa | XP_002301953.1 | cinnamyl alcohol dehydrogenase-like protein [Populus trichocarpa] | 6.0e-12 | 76% |
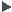 Full-Lengther Next Prediction Full-Lengther Next Prediction |
|---|
Fln status: Internal
Fln database: coniferopsida.fasta
Fln subject: C0PS97
Fln msg: Distance to subject end: 128 aas, your sequence is shorter than subject: 54 - 366
Fln protein:
T
Protein Length:
55
Fln nts:
T
Fln Alignment:
AllPine_a_rep_c92801___TEPGKSXXXXXXXXXGHMAVKFGKAFGLQVTVISTSPNKEKEAREVLEADHFLI
C0PS97________________TEPGKSLGVVGLGGLGHMAVKLGKAFGLRVTVISTSPKKEKEAREVLGADHFII

Biología Molecular y Biotecnología de Plantas, Facultad de Ciencias y Plataforma Andaluza de Bioinformática, Universidad de Málaga, E-29071 Málaga, Spain
