SERVICES
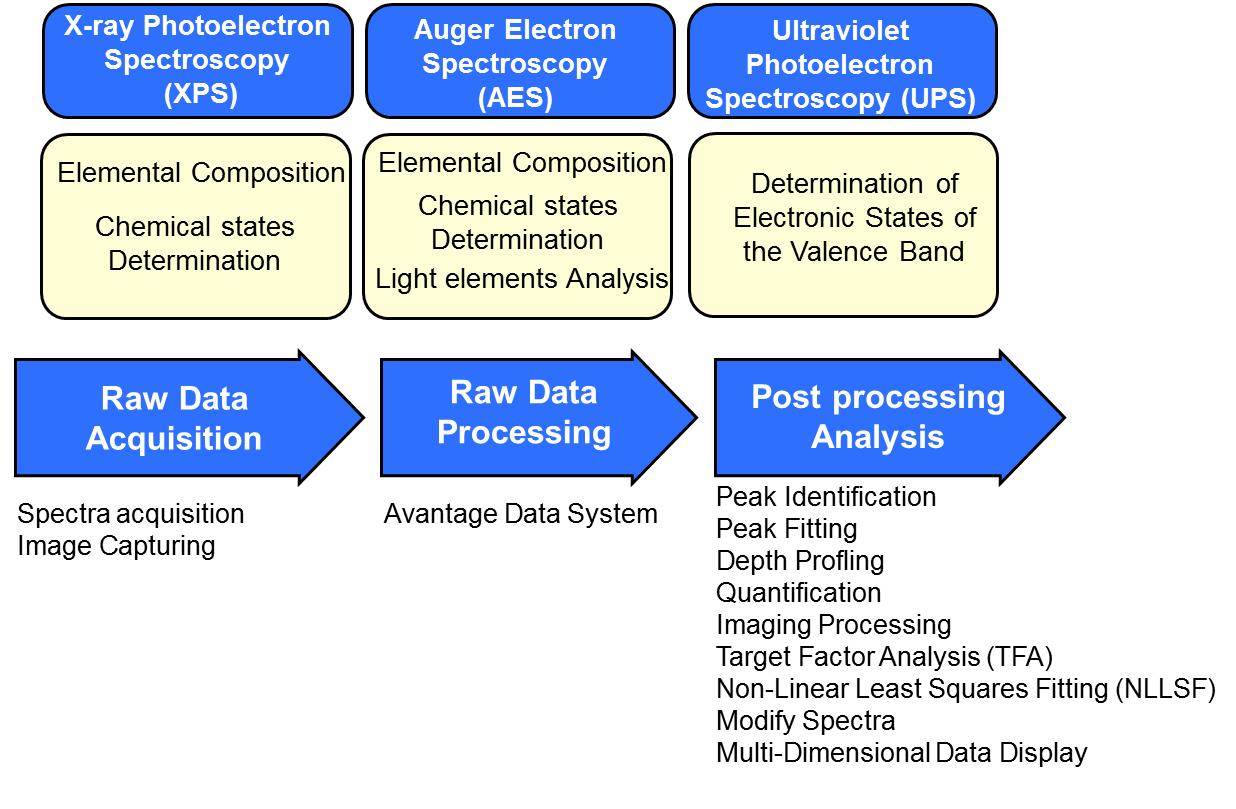
X-ray Photoelectron Spectroscopy (XPS)
Auger Electron Spectroscopy (AES)
Ultraviolet Photoelectron Spectroscopy (UPS)

X-ray Photoelectron Spectroscopy (XPS)
Auger Electron Spectroscopy (AES)
Ultraviolet Photoelectron Spectroscopy (UPS)
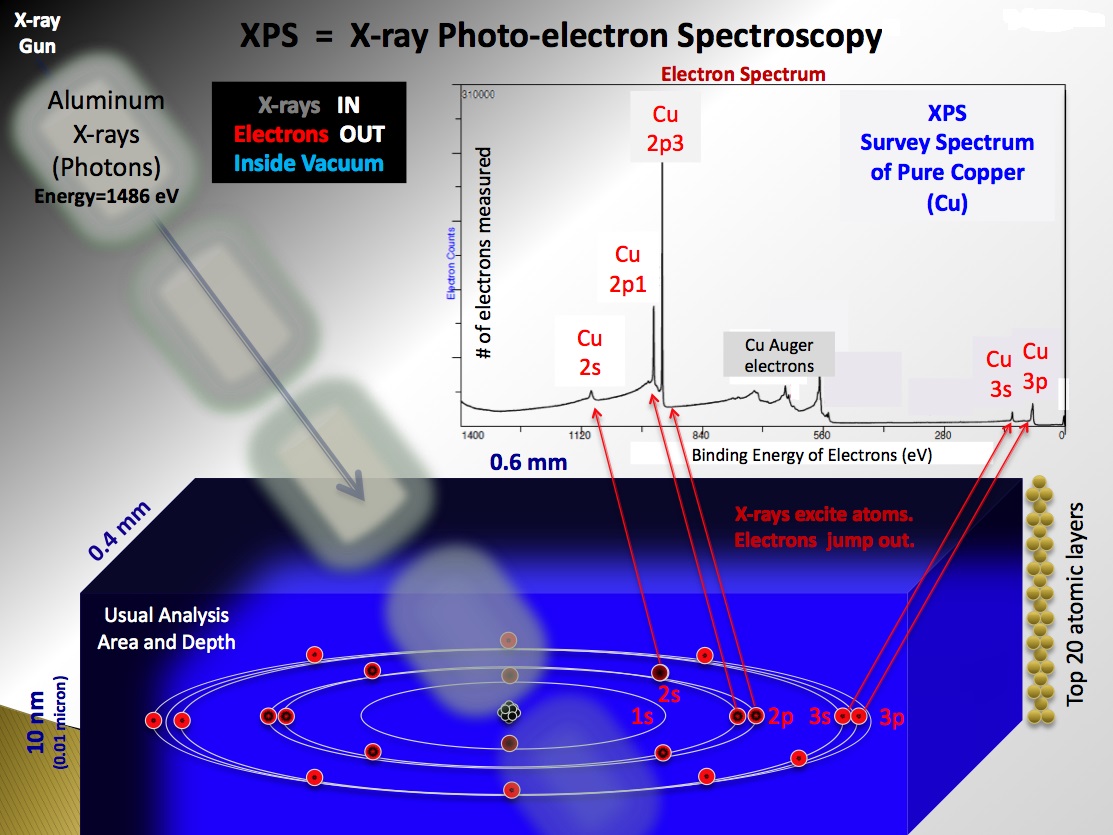
X-ray Photoelectron Spectroscopy is a surface-sensitive quantitative spectroscopic technique. In an XPS experiment, a specimen is irradiated by low energy X-rays in an UHV ultra high vacuum. This causes photo-ionisation of the atoms at the specimen´s surface: photoelectron are emitted from energy levels determined by the electronic structure of the specimen. The XPS analysis technique examined the kinetic energies and number of these photoelectrons that escape from the top 0 to 12 nm of the material analyzed to determine their energy distribution. From the results of this analysis it is possible to infer which elements are present on the speciment, what chemical states are, and in what quantities they are present.
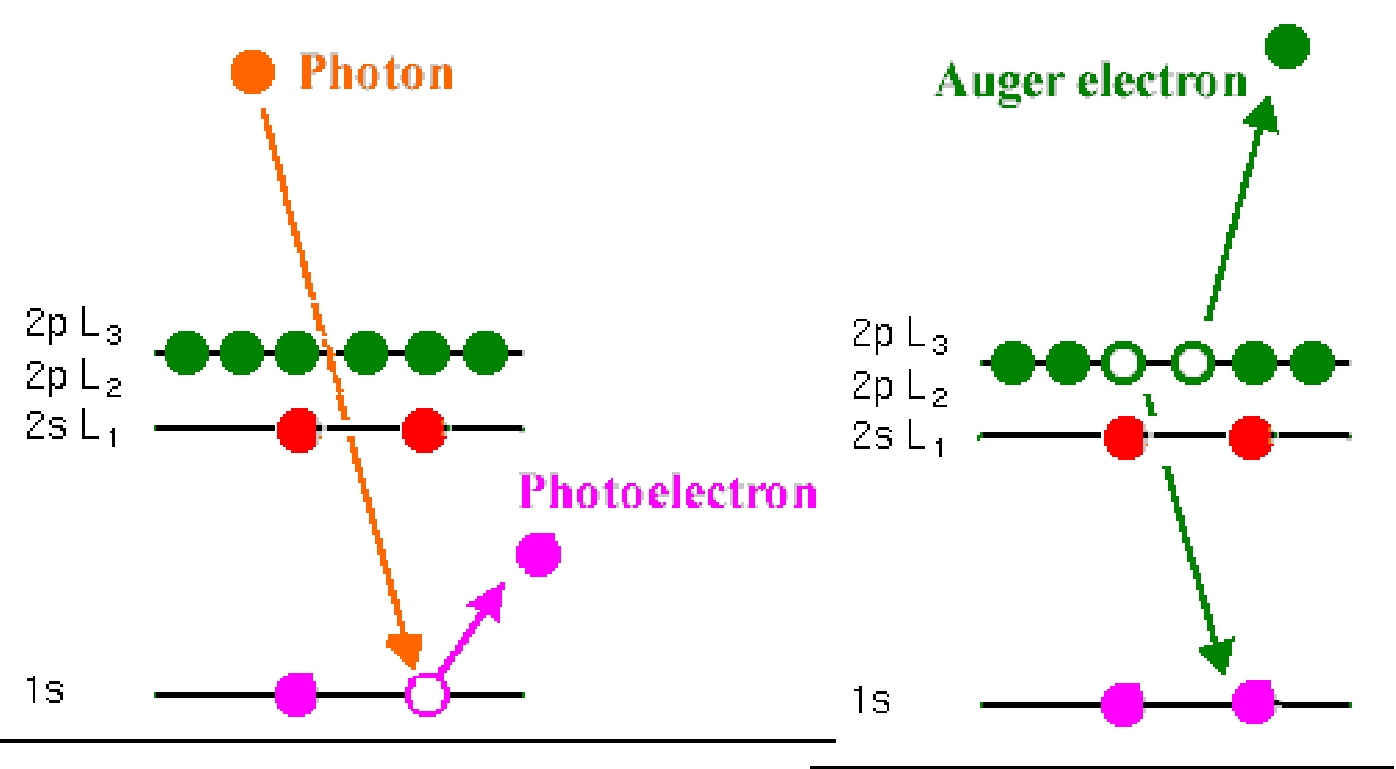 In an experiment AES atoms in a specimen also become ionised as a result of photoelectron emission; in this state the atomic structure contains an excess of energy after the photoelectron emission from the core levels, and will be relaxed by Auger electron emission. Each element produces a characteristic set of Auger peaks that indicates its presence. This technique is an efficient means for filling core holes of low binding energy, thus giving rise to relatively low kinetic energy Auger electrons with a short mean free path. Their detection outside the solid therefore provides a surface sensitive profile of elemental composition. In fact, this technique is the dominat mechanism for light elements, whereas XPS dominates for heavy elements.
In an experiment AES atoms in a specimen also become ionised as a result of photoelectron emission; in this state the atomic structure contains an excess of energy after the photoelectron emission from the core levels, and will be relaxed by Auger electron emission. Each element produces a characteristic set of Auger peaks that indicates its presence. This technique is an efficient means for filling core holes of low binding energy, thus giving rise to relatively low kinetic energy Auger electrons with a short mean free path. Their detection outside the solid therefore provides a surface sensitive profile of elemental composition. In fact, this technique is the dominat mechanism for light elements, whereas XPS dominates for heavy elements.
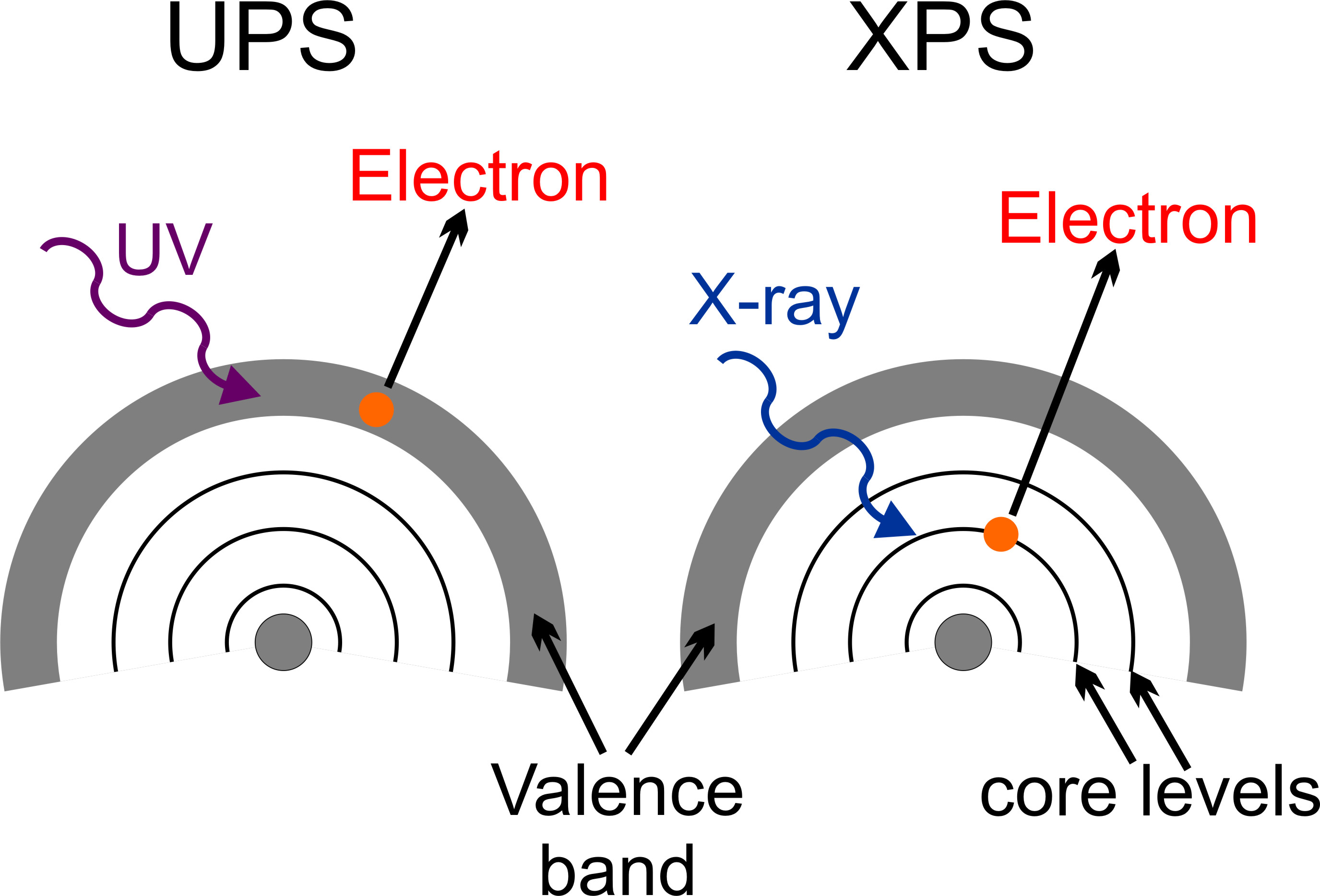 Ultraviolet photoelectron spectroscopy (UPS) refers to the measurement of kinetic energy spectra of photoelectrons emitted by atoms which have absorbed ultraviolet photons, in order to determine molecular energy levels in the valence region. This technique therefore is ideally suited to providing information about the more weakly bound and less localised valence electronic states of surfaces. This also makes it a valuable means for studying the surface band structures of clean metals and ordered atomic adsorption layers on metals. Information about the nature of a molecule´s interaction with a surface, such as bonding site, decomposition, orientation and interaction with other adsorbed species can be attained using the technique.
Ultraviolet photoelectron spectroscopy (UPS) refers to the measurement of kinetic energy spectra of photoelectrons emitted by atoms which have absorbed ultraviolet photons, in order to determine molecular energy levels in the valence region. This technique therefore is ideally suited to providing information about the more weakly bound and less localised valence electronic states of surfaces. This also makes it a valuable means for studying the surface band structures of clean metals and ordered atomic adsorption layers on metals. Information about the nature of a molecule´s interaction with a surface, such as bonding site, decomposition, orientation and interaction with other adsorbed species can be attained using the technique.